How important are catalytically active metals for us? And why don’t we know anything about them?
From our series: Colloidal Metals
Catalytically active metals play a special role in our body: we need them for specific metabolic processes – whether as building blocks in organs and tissues, as basic building blocks for hormone production or as central atoms in enzyme compounds.
In the case of metals, a distinction is made between highly catalytically active metals and less catalytically active metals. We know 83 chemically stable elements from the periodic table – but as the elements 43 (technetium) and 61 (promethium) have no stable isotopes, a total of 81 stable elements remain on earth.
The more catalytically active a metal is, the easier it is for chemical substances to react with each other, because the body itself has to expend less of its own energy – to put it simply. The aim here is to minimise energy consumption in reactions, a fundamental economic principle of life = no waste of energy!
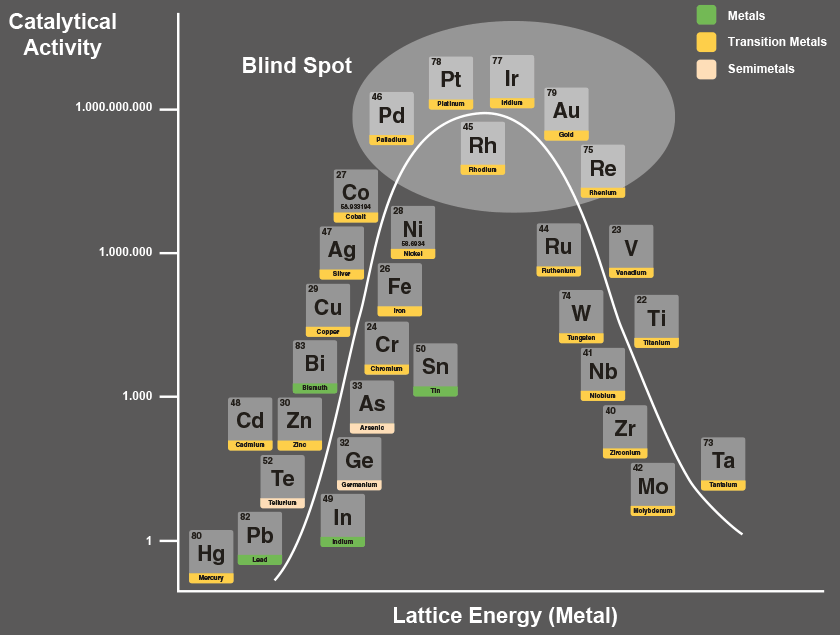
Volcano curve of catalytically active metals
The volcano curve of catalytically active metals (diagram) shows which metals can react as catalysts and how well. Catalytic activity always also means enzymatic activity. The difference between the degree of catalytic activity of mercury (Hg) and platinum (Pt) is one billion.
This means that due to its very high catalytic activity, platinum is a very important metal for our metabolism. It plays an important role as a central atom in enzymes. However, this has so far been mostly ignored in nutrition, food supplements and ecotrophology.
Catalytically active metals: Blind spot for analysis and evaluation
When looking at the catalytically highly active metals, it becomes clear that the ecotrophologists in particular seem to act according to the motto “What I don’t know or can’t analyse doesn’t exist or has no significance”. And accordingly, their recommendations lack any reference to the importance of these metals.
The reason is simple: the elements in the “blind spot” – platinum, iridium, palladium, gold, rhodium and rhenium – are difficult to dissolve. This is because the atoms of these metals are held together very tightly due to their high lattice energy. A great deal of effort is required to “break up” this lattice (spatial structure).
As a result, no attention is paid to these catalytically highly active metals when analysing foods and ingredients. The elements in the “blind spot” are not analysed and are therefore not considered essential. This is why they are gradually being removed from our food cycle. They are even being banned as a precautionary measure at EU level, while in the USA, for example, they can be bought everywhere as food supplements.
A lack of metals in our food leads to bio-logical impairments
If these metals no longer find their way into our food cycle in the required quantities as trace elements – via the water, the soil (years of monoculture and depleted soils), the plants (can no longer absorb anything from the soil), animals and ultimately our food – many enzyme compounds cannot be produced and work as they should. The body has to improvise more and more and incorporate “inferior” existing metals into the enzyme compounds as a substitute.
In contrast to an iron central atom, an enzyme with platinum in its centre has a significantly higher catalytic activity – by a factor of 1,000 to 10,000, see diagram. In the long term, the lack of catalytically active metals in the body leads to more and more dysfunctions in metabolism / cell metabolism and limited repair processes – from a bio-logical point of view, this is only a matter of time.
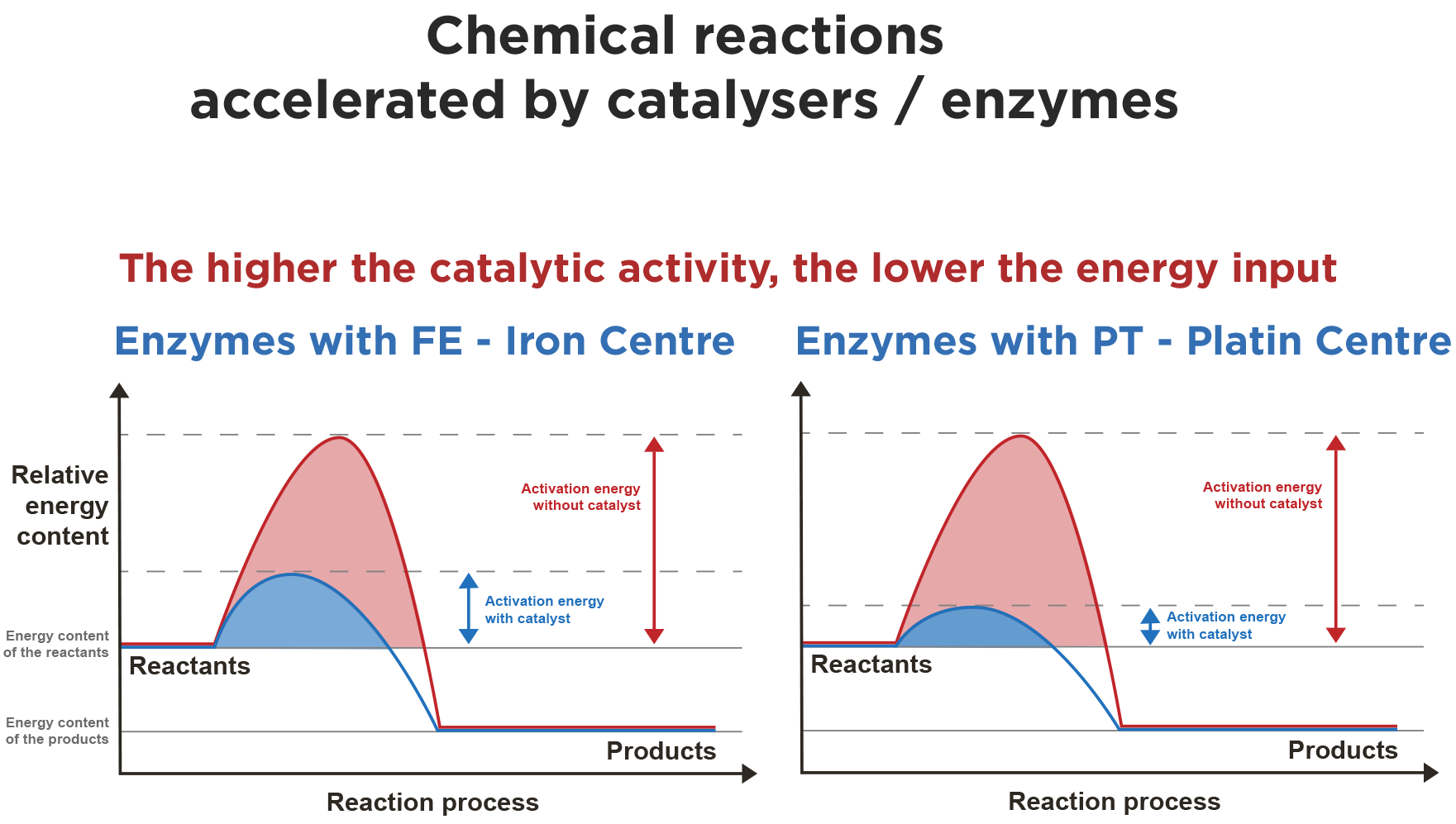
A certain level of reaction energy must be available so that starting materials can react. The energy must be made available in the body during metabolic processes (energy is consumed in the process).
Enzymes make it possible to reduce the level of necessary reaction energy. They act as accelerators and catalysers. As enzymes have different central atoms/metals in the centre, enzymes can reduce the necessary reaction energy slightly or also very strongly.
The catalytically active metals (blind spot of the volcano curve) in enzymes therefore only require a small amount of additional energy from the body in order for the chemical reactions to take place.
As our body works very economically, it will always try to work via enzymes with catalytically active metals in the centre rather than with less catalytically active metals – however, these elements must be present for this to happen.