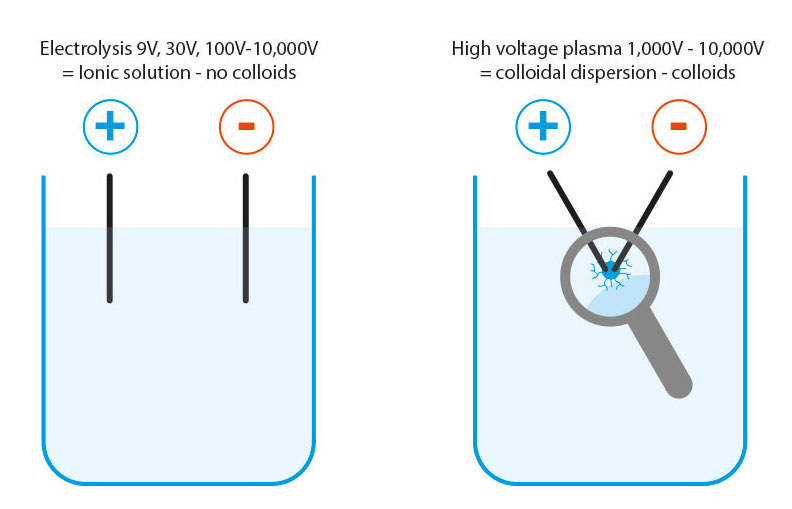
Clarification: Colloidal metals – ionic solution and colloidal dispersion are not the same!
Colloids are defined as suspended particles in a dispersion. However, the electrolysis manufacturing process produces ionic solutions (electrically charged and reactive metal ions). No matter whether they are produced with 9V, 30V or 10,000V, they are and remain ionic solutions! Compared to ionic solutions, colloidal metals produced in the high-voltage plasma process are real colloids in a dispersion.
Since there is a lot of contradictory information on this topic, we would like to shed some light on the subject with this article and clearly point out the differences between the two manufacturing processes electrolysis and high-voltage plasma. Since the manufacturing process has a significant influence on the results, possible risks and side effects, it is important to inform oneself precisely and to understand in detail what exactly happens and is manufactured in which process.
Elektrolysis: The process for producing ionic solutions does not produce colloids by definition!
- Electrolysis in the 9 to 60 volt range (“silver generators”)
- High-voltage electrolysis process, 100 to 10,000 volts
In electrolysis, the electrodes with a purity of 99.5 % to 99.99 % (silver, gold, platinum, zinc, copper, etc.) are placed in parallel in the purest water (optimally less than 1 μS). The DC voltage is applied with a plus and minus pole (9,000 to 10,000 volts depending on the device). Metal ions detach from the metal electrodes and go into solution with the water = ionic solution. Depending on the duration of the production and the voltage/current used, the concentration of the ionic solution increases, measurable in ppm (parts per million). Usually there is also a colour change, which is specific to each metal. The longer the production time, the higher the concentration of the ions in ppm.
The higher the lattice energy of a metal is, the higher the voltage must be to be able to dissolve ions from the metal electrodes at all. High lattice energy means that a lot of energy (higher voltages) must be used to dissolve ions from the metal lattice. With 9V battery systems, the production of e.g. gold water is hardly possible, because 9 V are not sufficient to dissolve enough gold ions in a reasonable time.
High-voltage plasma manufacturing: colloidal dispersion = true colloids
- Voltages from 1,000 to 10,000 volts: colloid production by means of plasma (electric arc)
In this process, the purest water (double distilled) is a basic requirement (value 0.5 μS) in order to obtain a plasma flame at all, which can dissolve colloids out of the electrodes. After atoms, colloids are the next largest particles with the same properties as the atoms themselves.
Even with the hardest metals, this plasma dissolves the smallest colloids from the electrode material with very high energy, which immediately condense in the water and cool down. These smallest colloids float in the water and do not settle. Part of the energy (10,000 volts) is transferred to the colloids themselves, so that these colloids are very energetic but electrically neutral.
Differences between electrolysis and high-voltage plasma processes
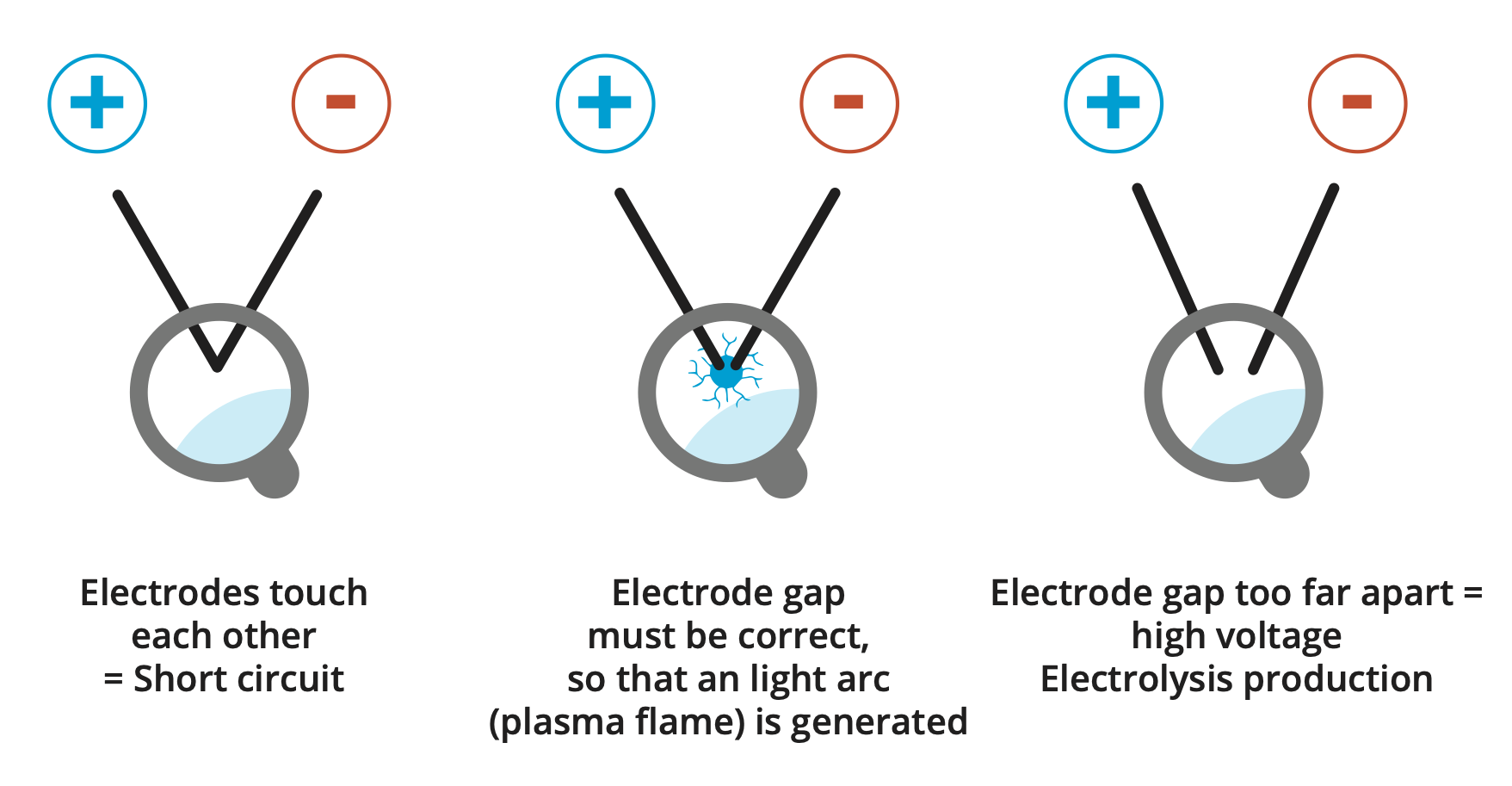
Automation is not possible with the high-voltage plasma process!
The 10,000 volts cause small to stronger vibrations at the electrode tips, which slightly change the position of the electrodes in relation to each other, which must be readjusted immediately!
The perfect water for colloid production
For colloid production, only osmosis-filtered water with subsequent double steam distillation (bi-distilled) with 0.5 μS should be used, as this is the only way to remove all substances from the water. Only steam distillation is not sufficient, as substances such as benzenes, chlorine or chlorine degradation products have a lower boiling point than water and would thus already be collected in the collection tank before the boiling point of the water and thus contaminate the distilled water.
During electrolysis production, residues and substances in the water could form direct compounds with the charged and reactive ions and produce compounds that are harmful to health, because reactive ions react quickly!
In high-voltage plasma manufacturing, residual substances would prevent plasma production because the current would flow directly through the water, which would prevent the plasma from forming.
Concentration in ppm or mg/l
The offer on the internet is huge and various concentrations are advertised. Mostly, the concentration is given in ppm (parts per million) or mg/l (milligrams per litre). However, the idea that “a lot helps a lot” is deceptive here, because a high concentration and the smallest energy-rich colloids are mutually exclusive: The higher the concentration, the more and larger clusters form, which significantly reduce the effectiveness and bioavailability of colloids. Concentrations of 5 to ≤ 10 ppm are optimal, have the smallest possible particle size and highest energetic charge.
The data in ppm can be equated with mg/l: ≤ 10 ppm correspond to approx. 10 mg/l.
The higher the concentration, the larger the particles or clusters: 5 to ≤ 10 ppm are very effective in their impact. The risk of clustering increases with higher concentrations, which means that the larger particles are then no longer as effective and cell-permeable or bioavailable. In the high-voltage plasma process, the particles are very small (1 to 20 nm) and have a very high particle energy, because the high energy (10,000 volts and temperatures between 3,000 and 4,000 degrees Celsius as plasma) needed to dissolve them out of the metal (lattice energy) is partly transferred to the resulting colloid particles. IIt is therefore better to take a silver colloid with ≤ 10 ppm twice than to take one with 20 or even 50 ppm once.
Conclusion:
Cheaper processes, such as chemical production (often relabelled as electrolysis because no one would buy it otherwise) or low-voltage electrolysis, are not a suitable production process, especially for gold or platinum colloid production, because of the high lattice energy. In addition, electrolysis only produces ionic solutions, by definition not colloids!
With the high-voltage plasma process, the best possible quality of colloids is achieved under economic conditions when produced correctly (smallest particles, highest particle charge, long shelf life, suspended state, no risk).
From our series: Colloidal Metals


